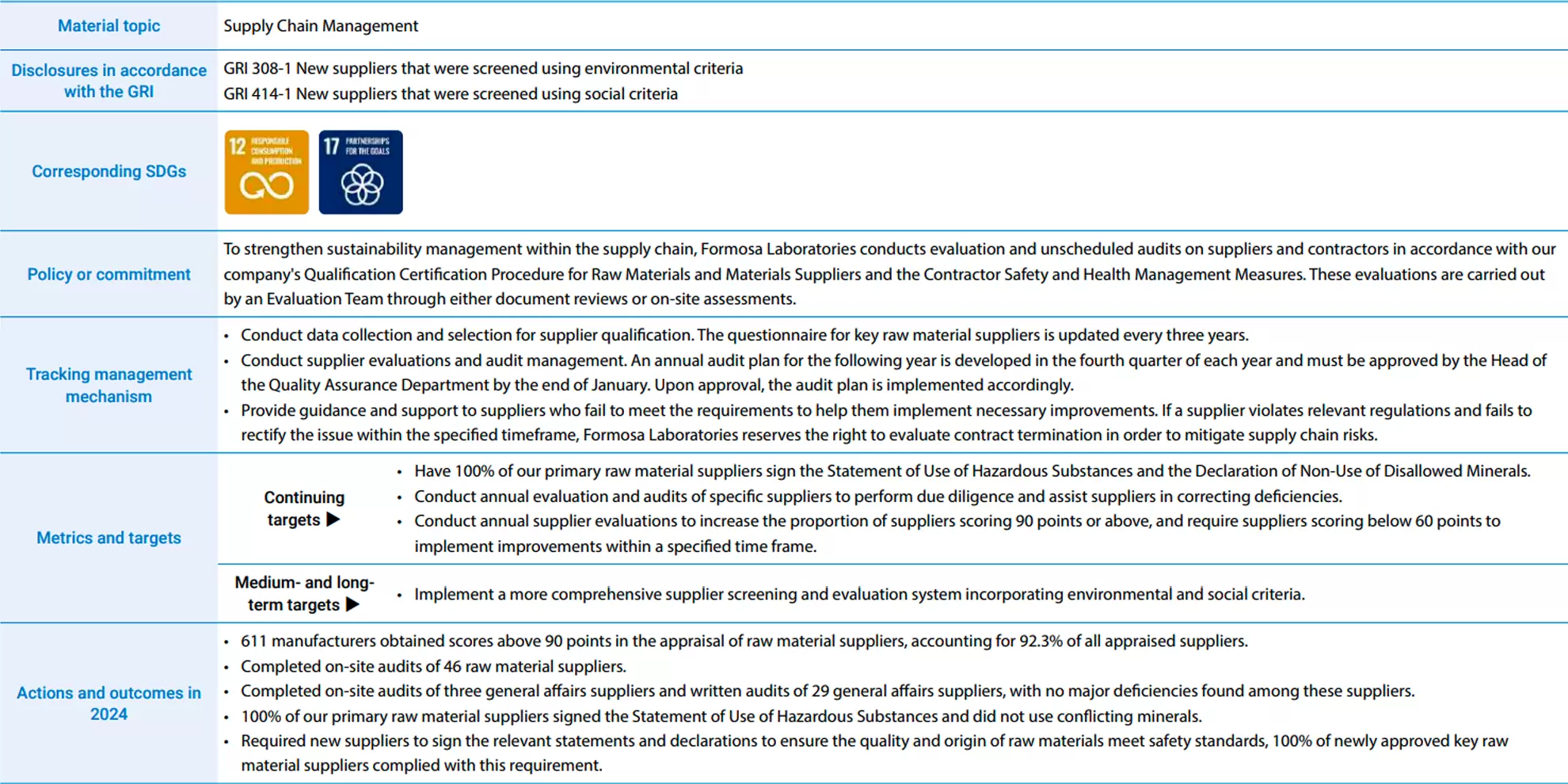
Supply Chain Management
Formosa Laboratories mainly develops manufacturing processes for APIs and Produces and sells APIs. In the biopharmaceutical industry, Formosa Laboratories is a specialized API manufacturer in the midstream sector, purchasing chemical raw materials and natural substances from suppliers and selling them to downstream pharmaceutical companies.
Committed to supply chain management, Formosa Laboratories conducts quality screening of new suppliers and regularly performs supplier risk assessments, evaluations, and audits to ensure raw material safety
▼Management of material topic“Supply Chain Management”at Formosa Laboratories in 2024

FORMOSA
FORMOSA
Supplier Risk Assessment
Besides requiring raw material suppliers to sign the Statement of Use of Hazardous Substances and the Declaration of Non-Use of Conflict Minerals, Formosa Laboratories follows our internal SOP for the Supplier Qualification and Assessment Certification Procedures. Simultaneously, this policy is included among the items required in supplier procurement management, as primary raw material suppliers must commit that the products or components they supply, along with their implementation of corporate governance and human rights protections for workers, align with Formosa Laboratories' ESG management philosophy. We also actively support suppliers that do not meet the requirements by implementing improvement programs. In case of any violation of relevant rules and regulations, Formosa Laboratories reserves the right to terminate or cancel our contract with the supplier to reduce supply chain risk.
Additionally, CO-202 Regulations Governing General Affairs Suppliers have been developed to manage the company's general affairs suppliers and ensure the stability and reasonableness of supply quality, quantity, prices, and so on.
Additionally, Formosa Laboratories continues to require new suppliers to sign relevant declarations and statements to ensure that the quality and source of raw materials meet safety standards and that they do not use unregulated hazardous substances. In 2024, 24 new suppliers signed these statements and declarations, representing 33% of all new suppliers for the year. Besides requiring suppliers to sign these documents, Formosa Laboratories completed the design of an ESG questionnaire for suppliers in 2024, including issues such as environmental protection, labor conditions, occupational health and safety, and human rights in the evaluation process. It is expected that questionnaires will be distributed to suppliers every three years starting in 2025, with the goal of establishing a more comprehensive supplier assessment system.
All of our primary raw material suppliers have signed the Statement of Use of Hazardous Substances, Halal Declaration, Allergen Declaration, Melamine Declaration, Genotoxic Impurity Declaration, and the Conflict Minerals Free Declaration.
Note : Primary raw materials refer to raw materials that can form the main structure of a product (API).

Supplier Evaluation
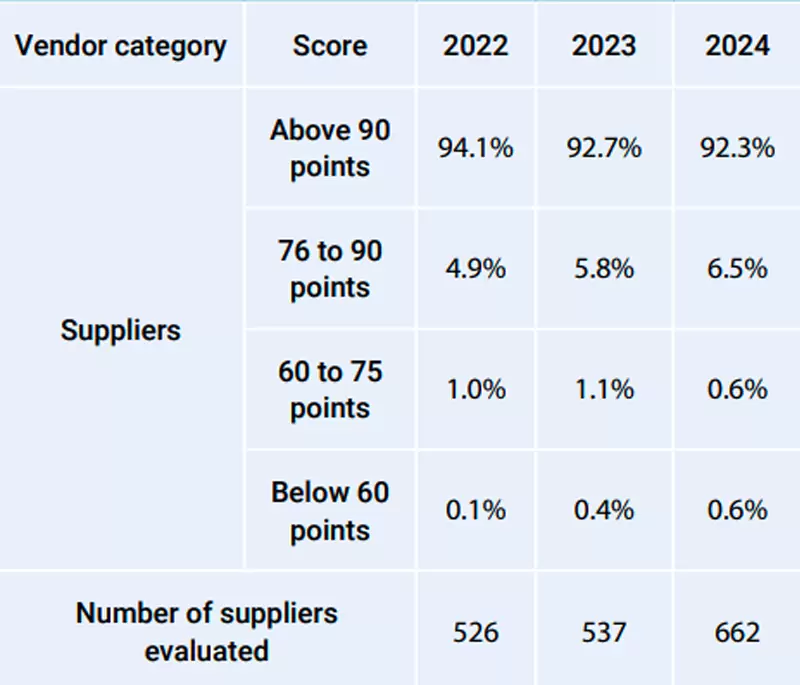
The Procurement Department conducts supplier evaluation according to the Raw Material and Material Supplier Evaluation Procedures. A supplier evaluation form is created regularly in the first quarter of the following year, based on the transaction history from the previous year. Weighting is assigned to each item in the evaluation form according to its impact on operations. Suppliers with scores below 60 points must make improvements within the specified time frame. Suppliers that fail to make necessary improvements will be disqualified from the vendor list.
▼Results of raw material supplier evaluations at Formosa Laboratories over the past three years

Supplier Audit
To ensure that all suppliers meet the company's requirements for workers' human rights, occupational health and safety, environmental protection measures, and other standards, the Procurement Department and the Quality Assurance Department develop an annual audit plan for raw material suppliers each year.
The Quality Assurance Department is responsible for conducting the audits to verify that the management and quality systems of the raw material suppliers comply with regulations. If any deficiencies are identified during the audits, suppliers must respond with corrective and improvement measures within one month of receiving the relevant audit report.
| Supplier category | Raw material suppliers | General affairs suppliers |
|---|---|---|
| Audit system | An annual supplier audit plan is scheduled in accordance with the Supplier Audit Management procedure, and audits are conducted in line with the plan. | The General Affairs Section is responsible for selecting, evaluating, and performing management assessment of general material suppliers of Formosa Laboratories according to Regulations Governing General Affairs Suppliers, including suppliers of group meals, drinking water, etc. Also, it randomly selects suppliers for on-site audits every year. |
| Audit results in 2024 |
|
|
▼Status of raw material supplier audits at Formosa Laboratories over the past three years
| Audit method Note 2 | 2022 | 2023 | 2024 |
|---|---|---|---|
| Expected number of suppliers audited on-site | 39 | 32 | 55 |
| Actual number of suppliers audited | 16 Note 1 | 20+5 (irregular) | 30+16 (irregular) |
| Achievement rate | 41.0% | 78.1% | 83.6% |
| Audit findings | No ineligible suppliers | One ineligible supplier. The cooperation with this supplier was suspended, and they were required to improve within a time limit. | Two ineligible suppliers. The cooperation with these suppliers was suspended, and they were required to improve within a time limit. |
Notes :
- The audit activities for this year were canceled due to different factors like the COVID-19 pandemic or the adjustment of the product development schedule in the plant.
- Information restatement: The information was restated due to misreporting in 2022 and 2023.