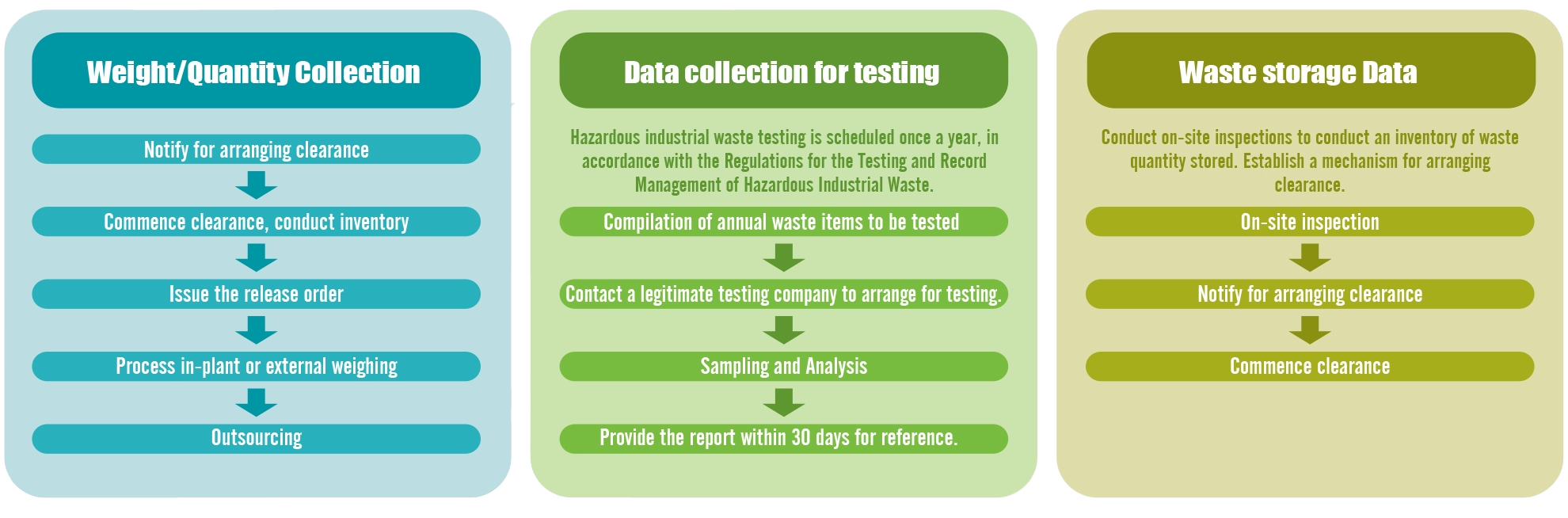
▼Management of material topic "Waste Management" at Formosa Laboratories in 2024
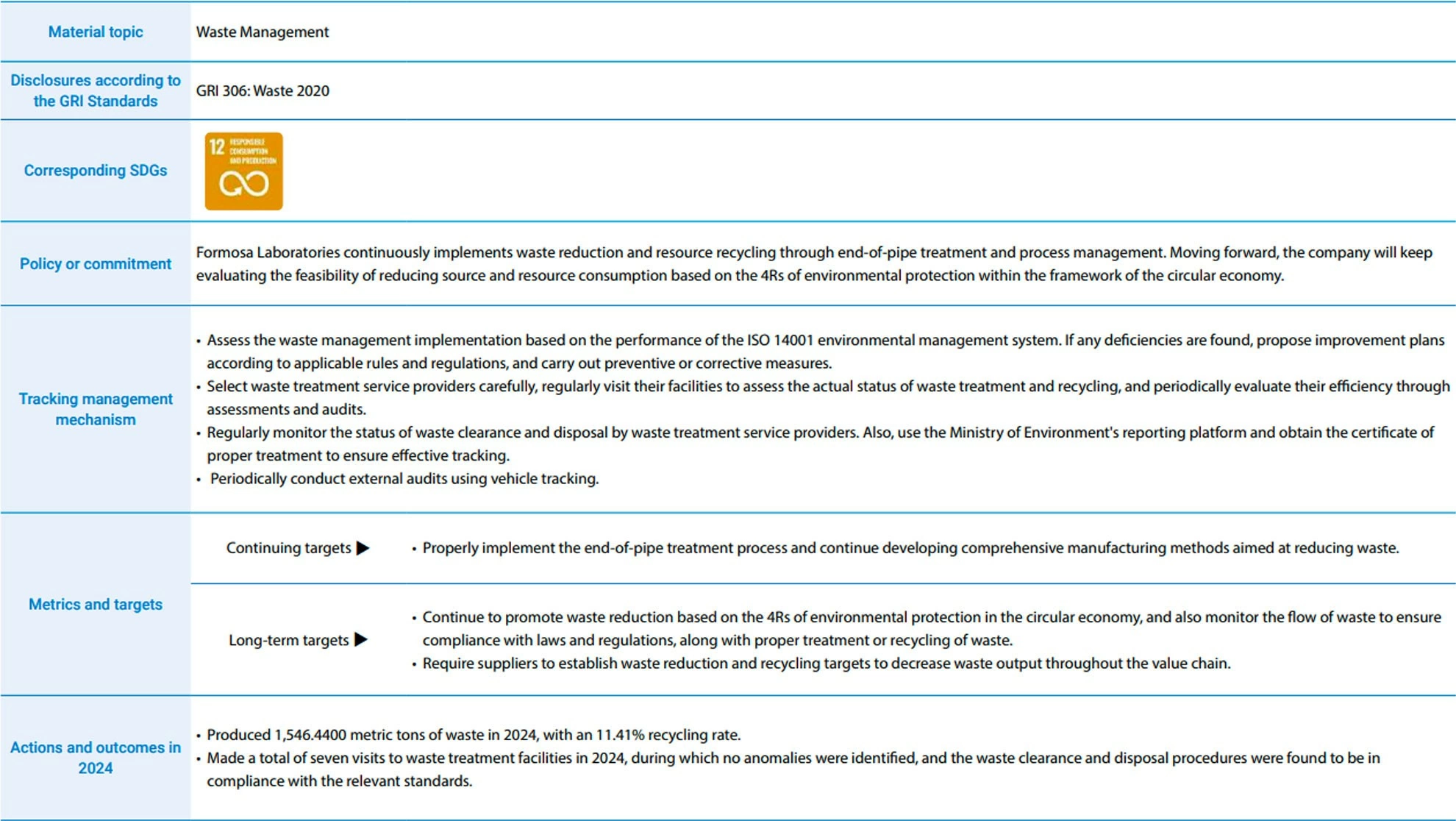
Waste Impact Management
All the industrial waste generated from pharmaceutical manufacturing at Formosa Laboratories is sorted, cleaned, and disposed of according to the Waste Disposal Act to prevent leaks during production, storage, transportation, and disposal. We also carefully choose waste clearance and disposal service providers and regularly conduct on-site audits. In 2024, Formosa Laboratories visited the waste treatment facilities of these providers seven times, finding no issues and confirming compliance with standards. For more information on waste impact analysis and management practices, please visit Formosa Laboratories' official website.
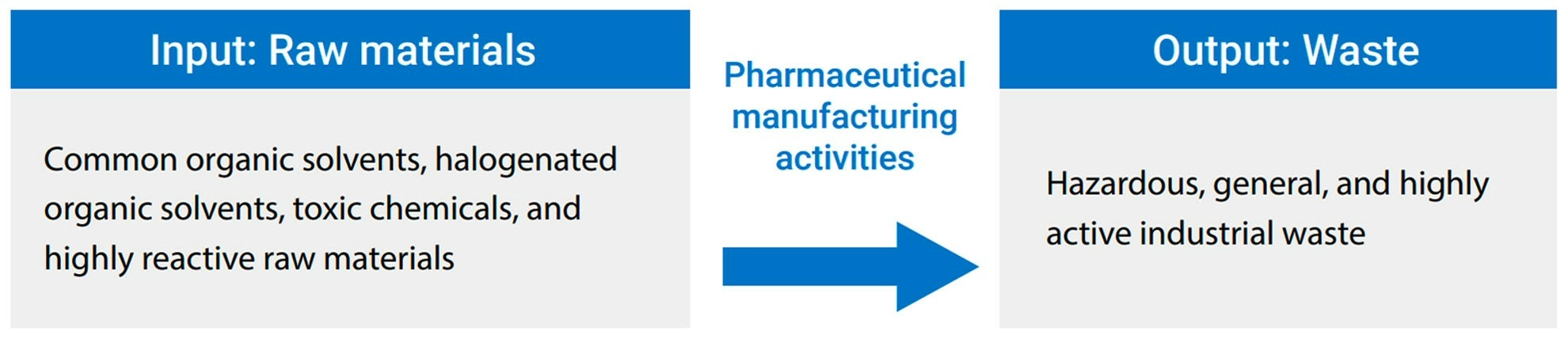
▼Waste production process at Formosa Laboratories
Waste Statistics
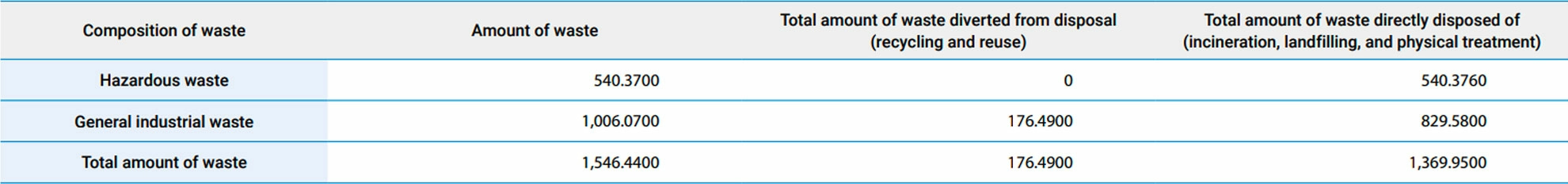
▼Total amount of waste at Formosa Laboratories in 2024 (Unit : metric tons)
Note:
- No waste was used in Formosa Laboratories' factories, as 176.4900 metric tons of waste diverted from disposal were recycled and reused.
- Of the waste directly disposed of from Formosa Laboratories, 1,019.5600 metric tons were treated by incineration, while 0 metric tons were landfilled, and an additional 350.3900 metric tons were treated by other means tons.
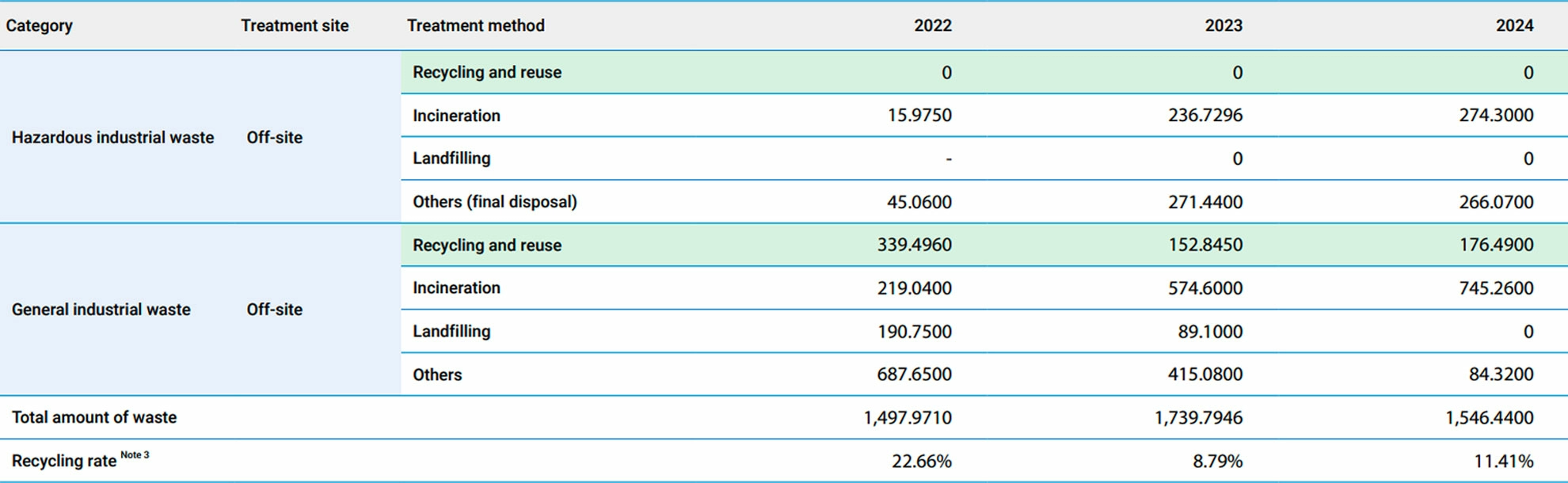
▼Status of Waste Treatment Summary Over the Past Three Years (Unit : metric tons)
 Note:
Note:- Treatment site : Off-site (outsourced treatment).
- Treatment method : Recycling and reuse (made into new materials through reprocessing), incineration, landfilling, and other disposal methods. 3. Recycling rate (%) = Total amount of waste recycled/Total amount of waste ✶ 100%.
- Hazardous industrial waste and general industrial waste are classified according to the Waste Disposal Act and the Standards for Defining Hazardous Industrial Waste in Taiwan.
- Information restatement : The information was restated to ensure all data had 4 decimal places and due to data misreporting from 2022 to 2023.
Collaborate with partners to reduce waste
As time passes, the issue of waste treatment plants reaching their deadline has surfaced, and the waste treatment problem will inevitably have an impact on the Company's sustainable operation. As a biopharmaceutical industry, Formosa Laboratories' production process generates three types of waste: hazardous industrial waste, non-hazardous industrial waste, and resource recycling waste. Adhering to the corporate sustainability development and environmental protection, the company promotes waste reduction and resource reuse through end-of-pipe treatment and process waste reduction. We continuously endeavor to care for the earth and work together to create a better environment for employees. In the future, we will assess the viability of source management in line with industry characteristic, life cycle concept, and the 4RS (Reduce, Reuse, Recycle, Recovery), considering measures such as source reduction and the use of recycled materials, as the basis for waste generation and control.
The waste produced by the Company is legally entrusted to qualified domestic suppliers for waste removal and disposal. In order to efficiently monitor waste flow and guarantee that all waste is lawfully and appropriately disposed or recycled, Formosa Laboratories meticulously selects waste removal and disposal supplier. We regularly visit the suppliers to verify and compare their actual waste treatment and reuse at their facilities. Also, we enhance the auditing effectiveness by requiring waste clearance companies to join the GPS system for tracking and management. In addition to effectively promoting waste management in our own operations and production processes, Formosa Laboratories requires suppliers to set goals for waste reduction and recycling to minimize waste production throughout the entire value chain. This fosters suppliers to jointly promote energy conservation, waste reduction, and circular economy.
Reducing the impact of waste pollution
Formosa Laboratories belongs to biopharmaceutical industry, mainly engaged in the development of APIs and the production of Generic Drugs. The technical processes involved in the development and production of APIS include crystallization, decolonization, filtration, extraction, concentration, drying, and recycling. As a result of the manufacturing process, hazardous industrial waste, hazardous liquid waste, non-hazardous liquid waste, waste solids, and high-activity waste are generated. Any negligence in production, storage, transportation, and disposal could lead to environmental pollution and impact. Therefore, we handle it with caution, classify and clear the waste in accordance with Waste Disposal Act to achieve compliance and minimize the impact.

Waste Input and Output Type at Formosa Laboratories
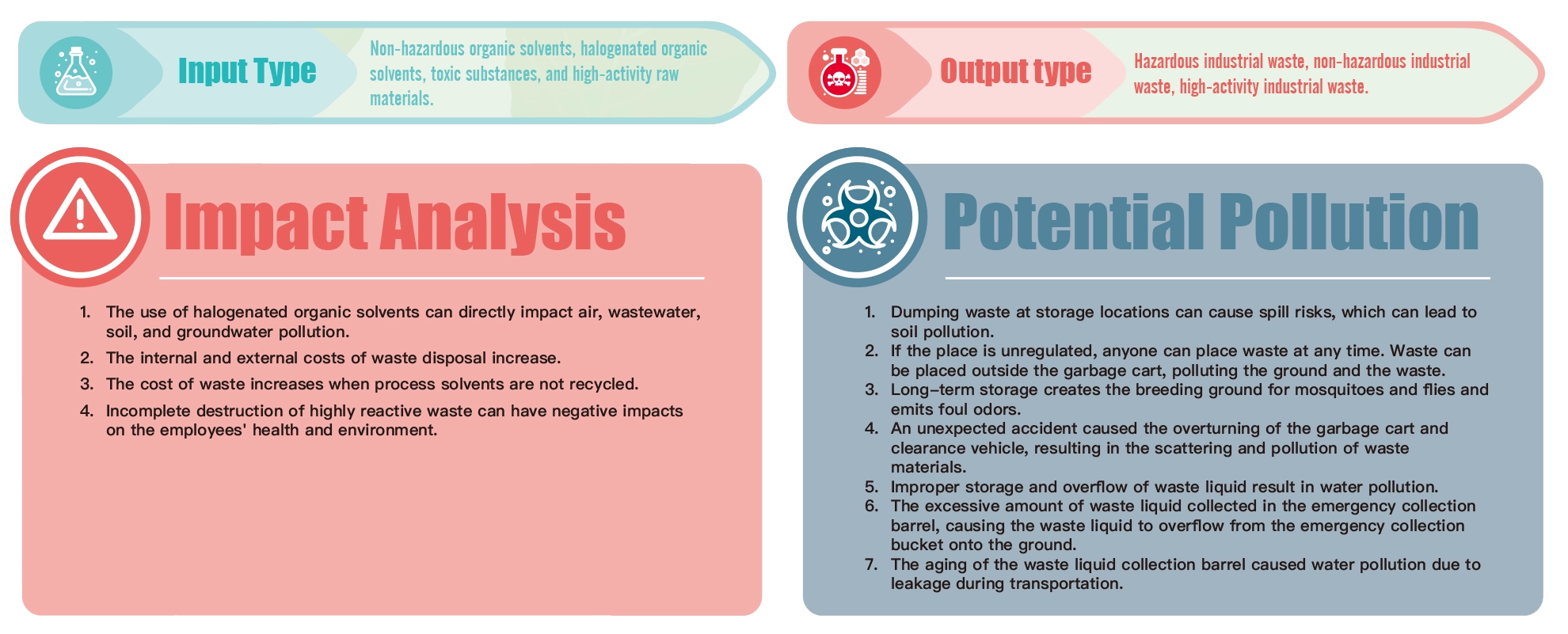
Waste Management System
- Classify and store waste according to its properties. The ground of the storage area should be made of reinforced concrete. Depending on the different properties of the waste, it should be packaged in a closed, barrel, bag, or other appropriate manner to prevent spill or exposure, which could cause hazardous reactions, odor generation, or environmental pollution such as water, soil, and air pollution. The goal is to provide a safe and healthy working environment for employees.
- Regularly inspect storage areas and promptly address any issues of spill, storage, or pollution.
- Implement high quality training to ensure that employees comprehend waste management and abnormal handling and reporting. Develop and enhance contingency mechanisms using internal communication channels.
- Waste generated from the upstream and downstream supply chain (such as waste generated from engineering projects in plant or raw material supply processes) should be classified and managed by the supplier as required in the contract for establishing a strong partnership between the upstream and downstream, and to create a win-win and sustainable cities and communities development.
- Promote waste reduction policies by using double-sided printing or reusing the back of paper whenever possible for official documents and paper usage. Use the photocopy paper brand that has FSC label and recycled container, encouraging manufacturers to recycle and reuse.
- Waste disposal is entrusted to suppliers who have obtained the waste clearance permit or reuse permit from governmental agencies. Through the declaration and confirmation process on the waste website of Ministry of Environment, the compliance of waste flow, disposal schedule, and method are ensured. If there is any abnormal risks, it will be reported through the Department of Environmental Protection's reporting process to ensure proper waste management.
- At least one inspection for clearance of waste items required by regulations is carried out and recorded annually to avoid the risk of illegal dumping.
- Maintain effective communication channels with the upstream and downstream supply chain to foster a partnership in achieving environmental protection goals (terrestrial ecosystem/marine resources). Continuously assess innovative treatment technologies, improve working conditions, promote economic growth, and create a sustainable world for Formosa Laboratories and Taiwan.

Review of Written Documents
- Through the declaration and confirmation process on the waste website of Ministry of Environment, verify the compliance of waste flow and process schedule. If there is any abnormal risk, report it rough the notification proc, so that we can do a good job in the basic management of waste materials.
- Irregularly conduct online audit on disposal suppliers for any illegal behaviors. If there are violations of environmental laws and regulations, a thorough understanding will be gained through on-site audits, and the number of surprise inspections will be increased.
Execution of Audits (on-site investigation or audit).
At least one inspection for clearance of waste items required by regulations is carried out and recorded annually. The relevant audits will be conducted in an unpredictable and early warning manner to avoid illegal dumping.
- Legal disposal suppliers that are certified by the Ministry of Environment.
- Possess a legal disposal permit.
- Clearance triplet manifest, GPS, weight note, proper clearance sheet, and in-plant release order.
- Ensure that the waste storage, clearance, disposal, and reuse are all in compliance with operational management and that records are maintained.
- Employer conducts visits and audits at least once a year and to keep a record of such activities.
Relevant Process of Collecting and Monitoring Data on Waste by Formosa Laboratories