Formosa Laboratories 2022 Material Topic: Innovative Research and Development
Research and Development Status
Formosa Laboratories is committed to providing innovative energy and assisting customers in expanding their business through its high level of expertise and research and development spirit. The Company works closely with customers to meet their needs, improve their research and production processes, and actively collaborate with customers and industry partners to enhance production efficiency and reduce the environmental impact of their products. In 2022, Formosa Laboratories' research and development expenses reached NT$471,194,000, accounting for 12.39% of its operating income, with a year-on-year increase of 1.30%. We were also granted 2 patents and have a total of 19 effective patents.

Investment in Research and Development by Formosa Laboratories in the Past 3 Years
| Year | 2020 | 2021 | 2022 |
|---|---|---|---|
| Investment Amount | 354,070 | 351,327 | 471,194 |
| Percentage of Revenue | 11.39% | 11.09% | 12.39% |
Research and Development Direction
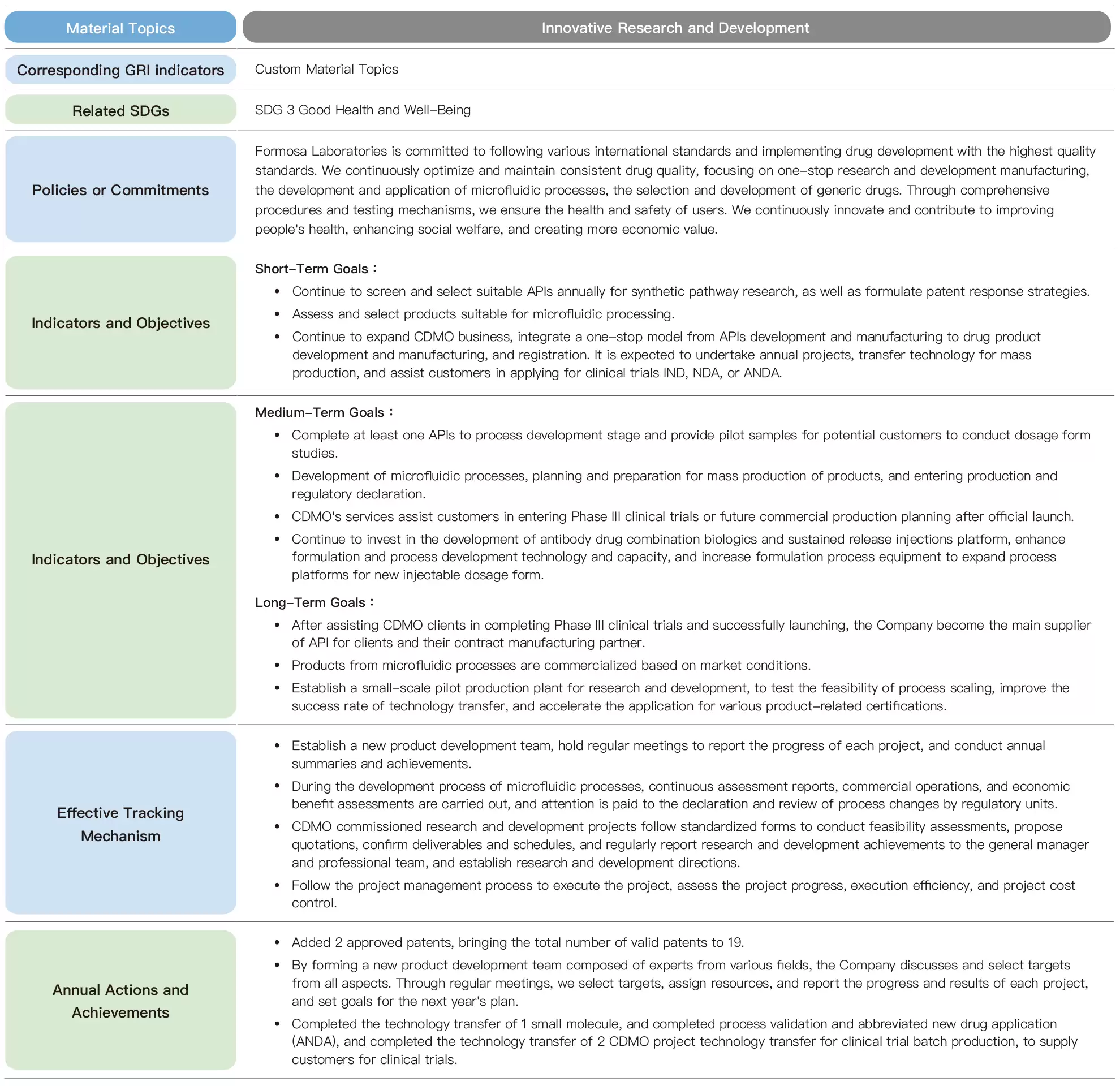
Formosa Laboratories is committed to comply with international standards and regulations, and through one-stop R&D and manufacturing, microfluidic process development and application, and the selection and development of generic drug labels, these three major projects will ensure the health and safety of people's use of medicines through well-established procedures and rigorous testing mechanisms, and will continue to contribute to the society through innovative research and development.
One-Stop Research and Development Manufacturing
Focusing on one-stop R&D and manufacturing, Formosa Laboratories is committed to follow Good x Practice (GxP) to perform drug development with the highest quality standards, develop stable and reliable formulations and process technologies, and continuously optimize and maintain consistent drug quality from R&D to production, from clinical trials to commercialization. Meanwhile, the Company provides innovative new drugs and technology platforms to contribute to the improvement of people's health, enhance social well-being and create more economic value.
The Injectable Department focuses on the one-stop development and manufacturing of sterile drugs, from small molecule or large molecule APIs to the development and production of drug formulations and injection preparations. The procedure of process development in the research laboratory and subsequent production manufacturing all complies with Good Scientific Practice (GSP) and Good Manufacturing Practice (GMP). Preparation development focuses on customized CDMO for high-difficulty synthetic technology APIs (non-biological complex drugs, peptide drugs, and antibody drug complex drugs, etc.), including formulation and process development, batch amplification, and submission batch production. The submissions include Investigational New Drug (IND), New Drug Application (NDA), Abbreviated New Drug Application (ANDA), Biologics License Applications (BLA), and all other items in the drug approval process.
Currently, our one-stop developed products include Eribulin and Gadoterate Meglumine, and FCM (Ferric Carboxymaltose) is under development. As for CDMO products, we provide small molecule drugs domestically and ADCs for international customers.
CDMO commissioned R&D projects follow standardized forms to conduct feasibility assessments, propose quotations, and confirm deliverables and schedules. Project execution follows the project management process to conduct project progress, execution efficiency management, and project cost control assessments. R&D projects regularly report research results or milestone plans to the General Manager and project team (including the R&D department, analysis procedure development department, Marketing & Sales Department, product and Project Management Department, and legal planning department), and jointly decide on the subsequent research direction.
The project team also regularly assesses the progress, execution efficiency and cost of each R&D project, and the overall average budget is within the expected control. In the past two years, we have completed one small molecule project with process validation and ANDA, and two biosimilar CDMO projects with clinical trial batch production for customers to perform clinical trials.
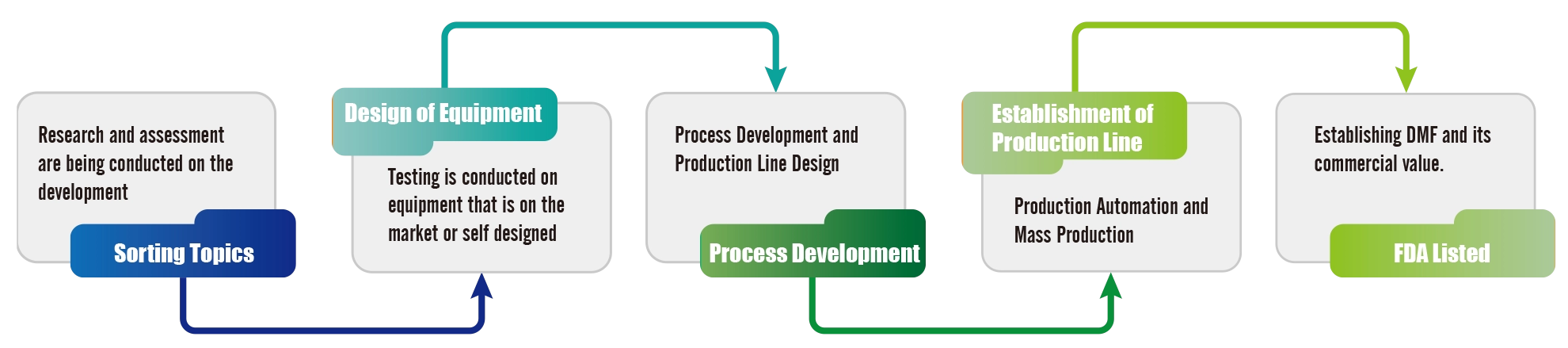
Formosa Laboratories Microfluidic Process Development Process
Development and Application of Microfluidic Processes
Microfluidics is a technology that miniaturizes fluids, and it can improve the efficiency of drug screening in micro and smaller dimensions. In drug development, it has lower production costs, requires smaller samples and reagent volumes, is faster, and takes up less space. It is an emerging field with great potential for development.
To meet the goals of carbon reduction, environmental protection, and sustainable operation, the development and application of microfluidic manufacturing are divided into short, medium, and long-term objectives. The short-term objective is to develop products suitable for microfluidic processes. The medium-term objective involves planning and preparing for mass production, along with production and regulatory declaration. The long-term objective is commercial operation. Throughout the process, microfluidic process development and assessment reports, commercial operation and economic benefit assessments will be carried out, and attention will be paid to changes in process and review by regulatory units. On an annual basis, initial microfluidic module construction and testing, mass production module construction and testing, and multiple evaluation reports will be conducted. Through a rigorous process and a comprehensive development product mechanism, establish health and safety guarantees are established for customers when using pharmaceuticals.
Selection and Process Development for Generic Drugs Target
After the expiration of the patent rights of the original pharmaceutical company, other pharmaceutical companies are allowed to produce approved drugs using the same ingredients and processes. In terms of the development and application of generic drugs, Formosa Laboratories sets short, medium, and long-term goals. The short-term goal is to select at least 5 targets each year for continuous screening and research on suitable APIs for synthesis pathways, as well as to formulate patent response strategies. The medium-term goal is to evaluate the selected targets, with at least 1 completed process development and provide pilot samples for potential customers to conduct dosage form studies. The long-term goal is to assist customers in completing phase III clinical trials and successfully launch the product, becoming the main supplier of APIs for customers and their contract manufacturing partner.
To achieve the goal,we established a New Product Development (NPD) team, we established a New Product Development (NPD) team comprised of experts in marketing, patent interpretation, chemical synthesis, manufacturing technology, experimental analysis, and supply chain construction, etc. to discuss and select targets from an allround perspective. Regular NPD meetings are held to report on the progress of each project, selection of targets, assignment of resources, and the progress of each project. The NPD team uses the annual summary to match the company's operation and market dynamics as a reference for future development direction.

Patent Layout
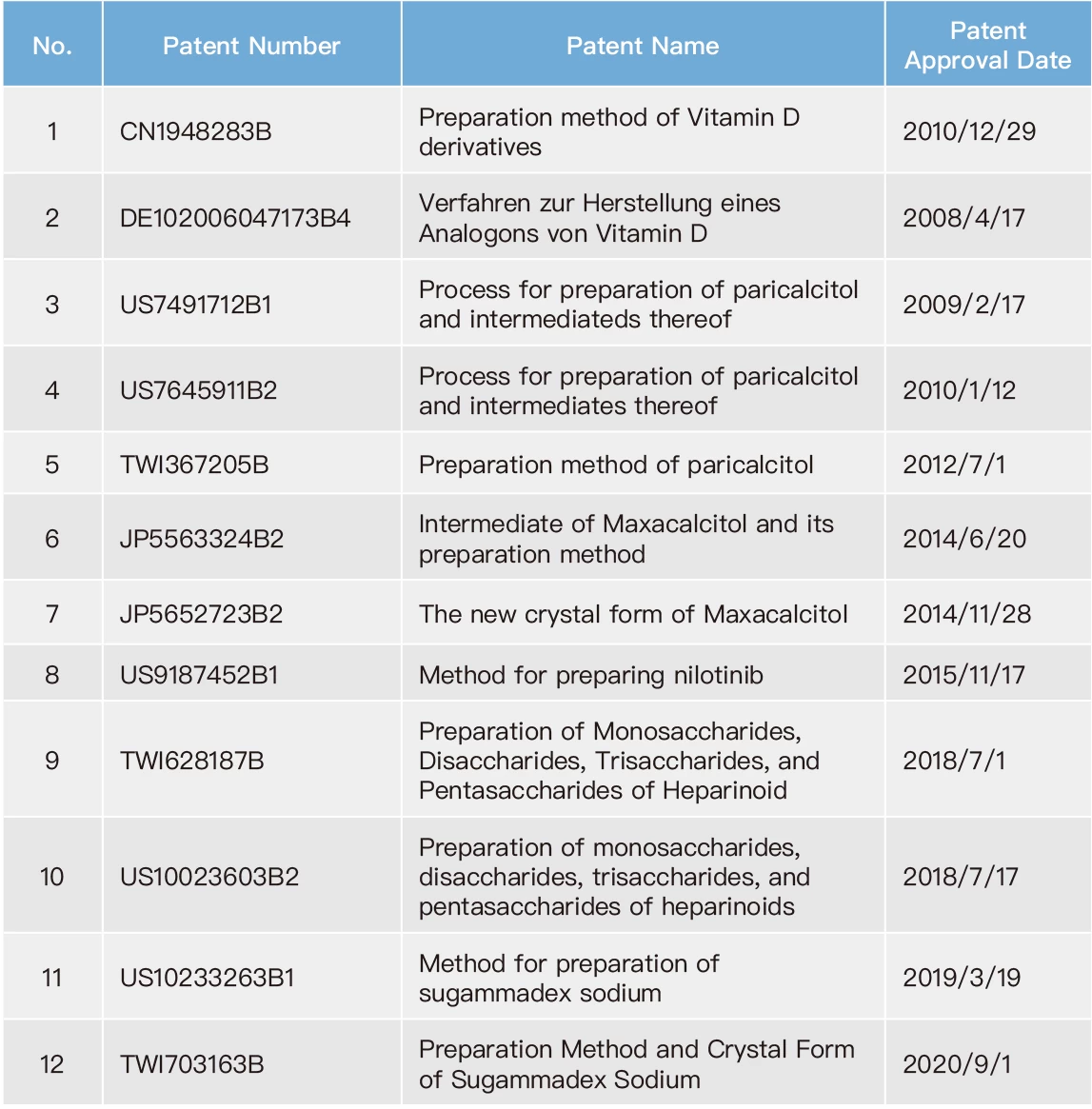
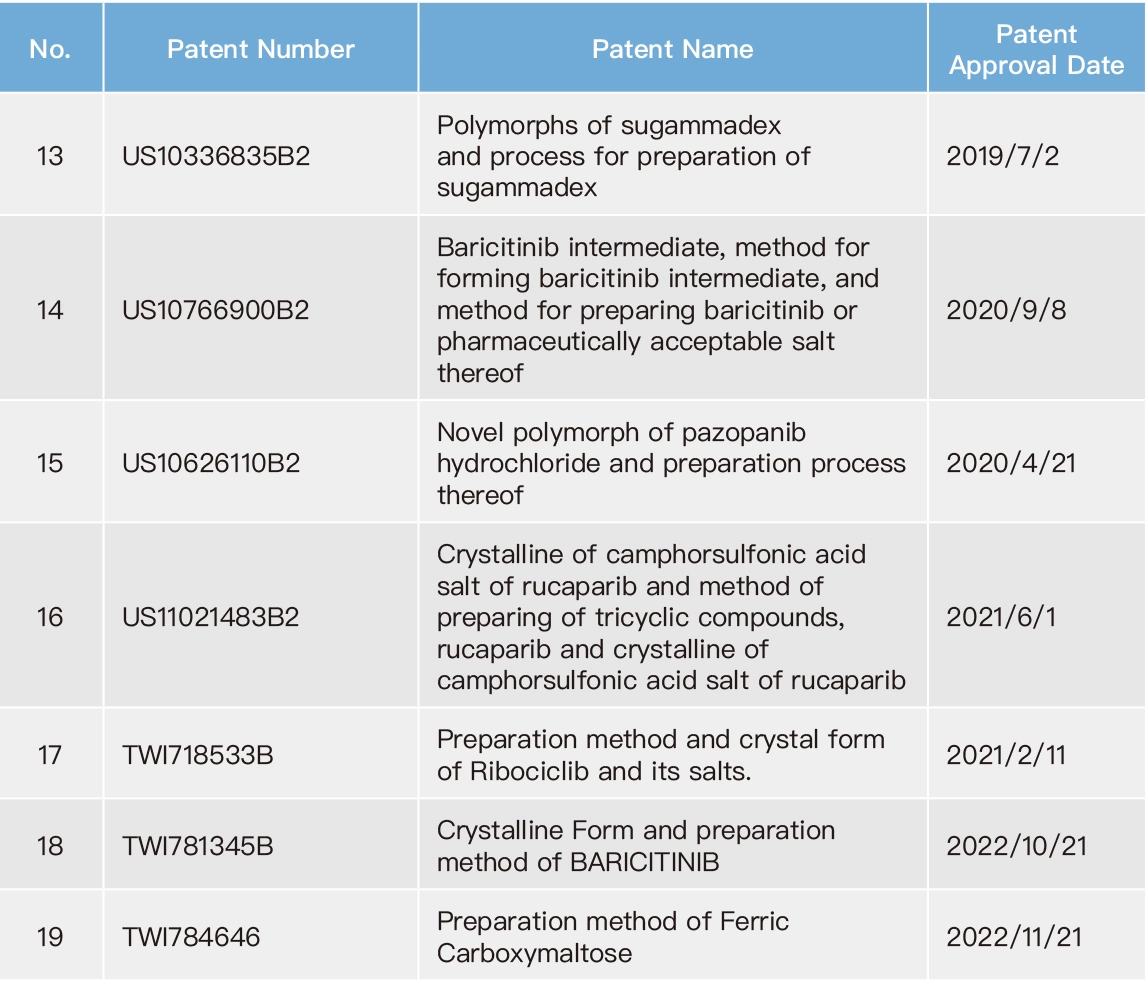
Due to competition in the pharmaceutical industry, it is common for the original drug developers and the generic drug manufacturers to engage in patent infringement lawsuits. Meanwhile, competition among generic drug manufacturers relies on subsequent patent applications as a means to gain an advantage. Therefore, Formosa Laboratories has dedicated patent engineers who conduct comprehensive patent search for different countries since the initiation of new product development, especially focusing on the markets in which the new products will be introduced. Patent engineers actively cooperate with our R&D to develop our own technology, to assure our manufacturing process not to infringe against competitors' patents. Our company have issued the company's "FTO Analysis Regulation”: as a basis to ensure the development of new products in a mature manner. On the other hand, our company also have issued the company's “Patent Application Regulation” to encourage colleagues to apply for patents and build a comprehensive patent layout for Formosa Laboratories. To date, Formosa Laboratories has produced 27 inventions. With careful reviewing global market competition and the Company's business layout as criteria, the patents from these inventions were determined whether to be maintained. Therefore, as of the end of 2022, Formosa Laboratories still maintains 19 valid patents.
Patent Acquisition Status of Formosa Laboratories in the Past 3 Years
| Country | New Patent Cases | Accumulated Number of Valid Patents as of 2022 | ||
|---|---|---|---|---|
| 2020 | 2021 | 2022 | ||
| Taiwan | 1 | 1 | 2 | 6 |
| USA | 2 | 1 | 0 | 9 |
| China | 0 | 0 | 0 | 1 |
| Germany | 0 | 0 | 0 | 1 |
| Japan | 0 | 0 | 0 | 2 |
| Total | 3 | 2 | 2 | 19 |
List of Valid Patents for Formosa Laboratories in 2022