Search
x
NEWS
PASSION
Product & Service
Contract Development and Manufacturing Organization

Product & Service
Products
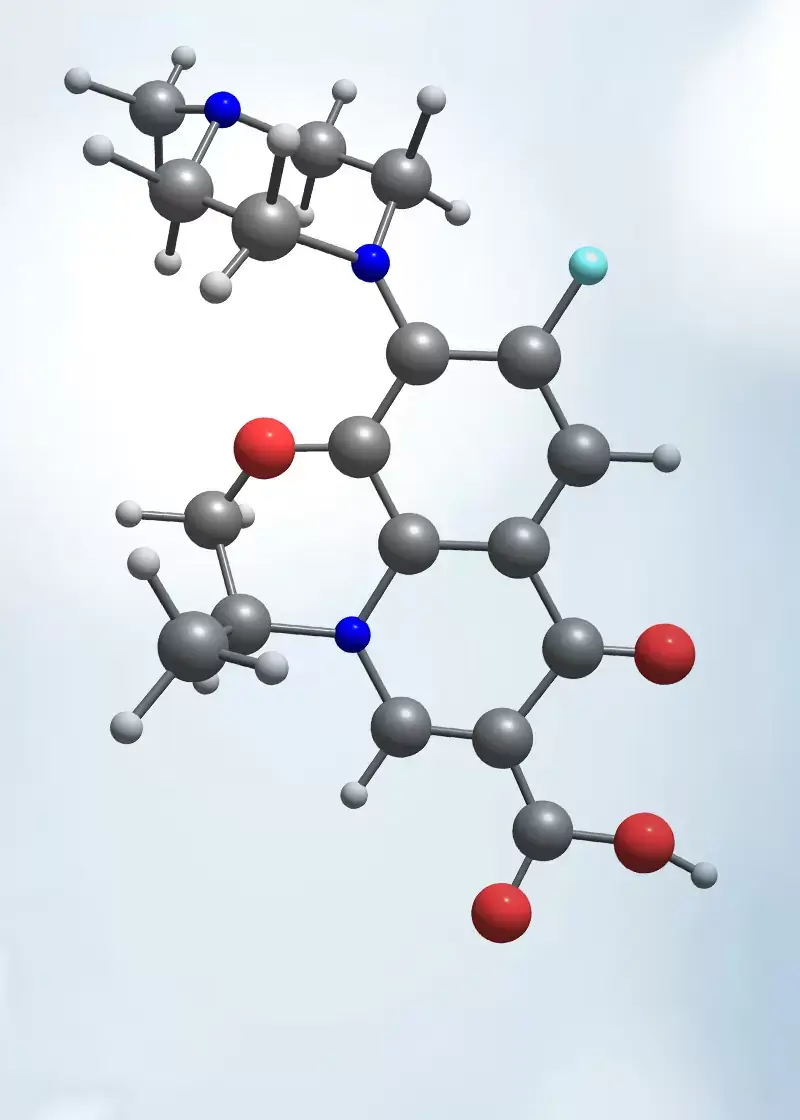
We develop and manufacture APIs and Injectables with state of the art technology under cGMP in compliance with the cGMP regulations.
INNOVATION
Sustainability
Quality & Compliance
GMP-Compliant
inspected by TFDA , FDA, EDQM,
and PMDA
Formosa Laboratories, Inc. commits itself to the manufactures of all its pharmaceutical products in accordance with the current Good Manufacturing Practices (ICH Q7) and by the methods ...




Hope

Sustainability
Create a better future for the next generation.
As a resident of the earth, Formosa Laboratories not only adheres to the strategy of corporate governance on truthfulness, transparency and integrity, but also participates in social and environmental activities, such as talent cultivation, energy conservation and carbon reduction. And enhance the sustainable force that keeps the society moving forward to become a corporate citizen with social impact and create a better future for the next generation.